usp class vi materials
A USP Class is a more granular classification occurring within a specific USP Category in the USP Drug Classification system. Forge Labs Bio Medical Clearvue is USP Class VI.

Material Selection Medical Injection Molding Xcentric Mold
Multi Jet Fusion MJF.

. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and. 27 rows The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. So does ISO 10993.
Reference to a specific partsection in the US 21 CFR or to an EC directive would be more meaningful. USP stands for US. Registered to ISO 9001 FEA design engineering Application engineering Material characterization Precision CNC machining.
FEP Shrink Tubing 131 161. Forge Labs Bio-Medical resin is an high clarity transparent resin that simulates the material properties of Polycarbonate. Yet some suppliers that use compliant ingredients may still not be able to guarantee a compliant end-product.
USP Class VI Testing Methods. That said the lack of risk assessment in USP Class VI can be a problem. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification.
They typically include not only the raw material conformity such as ISO 10993 and USP class VI but also the examination results of the semi finished product in accordance with ISO 10993. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. USP Class VI vs.
Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. Suitability under USP Class VI is typically a base requirement for medical device manufacturers. USP Class VI Approved Plastic Materials.
Class VI is the most stringent and requires. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. AdvantaFlex TPE Biopharma Tubing.
USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test. However its acceptability applicability is declining while ISO 10993 becomes the gold standard. With the order related documentation we ensure traceability from the customers order for the semi-finished product and the raw material used.
As one of the most widely used methods VI forms part of six different classes with this being the most thorough. This medical-grade material is a rigid non-brittle resin ideal for producing biocompatible parts that require long term skin contact and end-use medical devices. Excelon RNT 68 Food Beverage Tubing.
Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and other health care technologies. In order to identify the biocompatibility of materials USP Class VI testing is required. For plastics they have six different classes based on duration and application.
Excelon RNT 60 PVC Vinyl Lab Tubing. USP Class VI Gamma EtO sterilizable for medical. Typical applications for our FDA NSF 51 USDA materials are disposable medical devices surgical instruments and medical fluid dispensing components as well as a wide variety of food and beverage.
The material exhibits high elongation superior toughness durability and abrasion resistance. However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. Excellent strength thermal stability ability to withstand steam autoclaving NSF-51 food contact Bio-compatible ISO 10993USP Class VI.
Eventually the answers depend on where you are required to clear a device. Most applications are fairly benign to elastomers. Its possible that a USP Class VI material can also.
SIMONA PP-H USP Class VI sheet material is easy to clean and disinfect using most hospital grade cleaners and disinfectants. Moldable polyurethanes Resilon 4300 and 4301 Molythane 4615 Machinable polymer-filled 0618 PTFE Life Sciences Capabilites. C-Flex ULTRA biopharma pump tubing.
In USP DC 2022 there are 172 USP. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. FDA Food Drug Administration takes responsibility for determining whether and how manufactured materials may be used in contact with food products.
Definitions for proper use are found in a series of regulations published annually under. In USP DC 2022 there are 51 USP categories. In addition SIMONA PP-H USP Class VI sheet delivers high chemical and corrosion resistance.
This form of testing is designed to certify that no harmful reactions or long-term issues are caused to the body by chemicals that are. USP Class VI demands an intracutaneous irritation test. Excelon RNT 1065 Vinyl Tubing.
A USP Category is the broadest classification of the USP Drug Classification system and provides a high level formulary structure. FDA and USP Class VI O-ring materials for life sciences Parker Compound Polymer Hardness Color Temperature Range F Service EJ280-70 EPDM 70 Black -70 to 250 FDA USP VI Animal-free E3609-70 EPDM 70 Black -70 to 250 FDA USP VI FF156-75 FFKM 75 Black 5 to 525 Broad chemical resistance USP Class VI. USP class VI is also a good starting point.
Class testing is frequently conducted on plastic materials that come in contact with injectable drugs and other fluids found in various steps of the drug manufacturing process. Our USP Class VI certified material offering includes.

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Usp Class Plastics Pacific Biolabs
Dursan Passes Usp Class Vi Testing Why Is That Important
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

What Is Usp Class Vi Testing Tbl Plastics

Usp Class Vi Foster Corporation
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
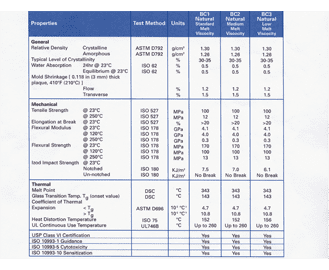
Meaning Of Usp Class Vi Standard United Kingdom

Meaning Of Usp Class Vi Standard United Kingdom

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Usp Class Vi And Biocompatibility Of Products For Pharmaceutical Use Mtg




